Business Hours: Monday - Friday: 9 AM - 6 PM EST

Cingal FDA Approval Status
David Fuller
Last Updated On: November 26, 2024
Knee injections are a widely popular treatment for osteoarthritis (OA), offering significant pain relief and improved mobility for millions of patients. Research shows that viscosupplementation, which involves injecting hyaluronic acid into the knee joint, can effectively reduce pain in up to 60% of cases. Despite their growing popularity, regulatory approval plays a crucial role in determining the accessibility of these treatments.
One notable option is Cingal, an innovative knee injection combining hyaluronic acid with a corticosteroid to deliver immediate and long-lasting pain relief. However, questions surrounding its FDA approval status have made it a topic of interest among patients and healthcare providers in the United States.
In this article, we will examine the status of Cingal FDA approval, discuss this treatment’s benefits, and explore its approval status for individuals seeking advanced solutions for OA symptom relief.
Key Takeaways
- Cingal injections combine hyaluronic acid and corticosteroids, providing immediate and long-lasting relief for knee osteoarthritis.
- The product is approved in over 35 countries, including Canada and many in Europe, but has not yet received FDA approval in the United States.
- Cingal has undergone rigorous clinical trials demonstrating its safety and efficacy, with relief lasting up to six months.
- The dual-action formulation distinguishes Cingal from other viscosupplements by addressing inflammation and joint lubrication.
- The ongoing FDA review process underscores the importance of regulatory compliance and safety for future availability in the U.S.
About: Operating since 2016, Med Supply Solutions is known for being one of the industry’s top and trusted suppliers of cosmetic and viscosupplementation products. Contact our sales department for more information about buying Cingal online.
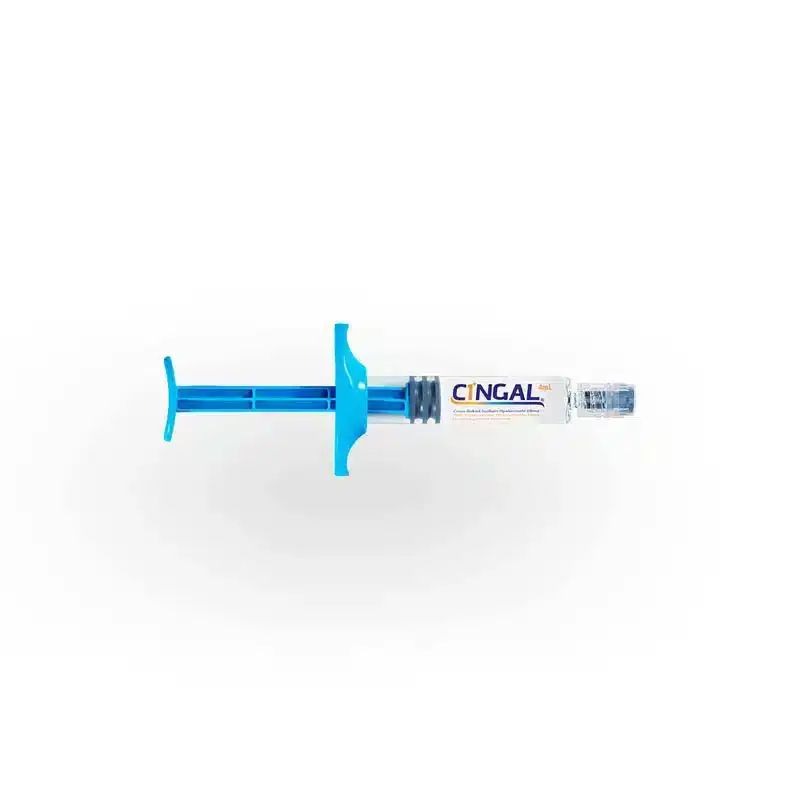
CINGAL®
Hyaluronic acid
TRIAMCINOLONE HEXACETONIDE
$249.00
Tier pricing
Save 4.02%
3 or more
$239.00 each
Save 6.02%
11 or more
$234.00 each
Save 8.03%
21 or more
$229.00 each
The Clinical Trials and Regulatory Process

The regulatory process for FDA approval requires manufacturers to provide comprehensive data from these trials, including evidence of safety, efficacy, and quality. This involves multiple phases:
- Phase I Trials: Assess initial safety in a small group of participants.
- Phase II Trials: Evaluate dosage levels and effectiveness in a larger population.
- Phase III Trials: Confirm efficacy and monitor side effects in diverse and larger patient groups.
Cingal underwent rigorous clinical testing to evaluate its efficacy and safety. Three Phase III clinical trials were conducted, involving a total of 368 patients with mild to moderate knee osteoarthritis. These trials were randomized, double-blind, placebo-controlled, and multi-center studies.
The primary endpoint was the change from baseline in knee pain as measured by the WOMAC Pain Score. Results showed that Cingal provided rapid pain relief within days and long-lasting relief up to 26 weeks, significantly outperforming saline and hyaluronic acid alone.
The FDA meticulously examines this data to ensure the product’s claims are substantiated and that it poses no undue risk to patients. Despite Anika Therapeutics’ adherence to this process, Cingal still needs to receive FDA approval for use in the United States.
Following the successful clinical trials, Cingal underwent a comprehensive regulatory review process. The data from the trials demonstrated Cingal’s superiority in pain relief and safety profile, leading to its approval in over 35 countries.
The company is currently working with the FDA to discuss the next steps for U.S. regulatory approval. The approval process involves submitting detailed trial data, safety information, and proposed labeling to the FDA for review and approval.
Cingal’s Efficacy and Formulation Compared to Other Viscosupplementation Treatments

Cingal stands out in the viscosupplementation market due to its unique dual-action formulation. It combines hyaluronic acid (HA), a well-established component for restoring joint lubrication and cushioning, with a corticosteroid, which provides immediate anti-inflammatory and pain-relieving effects. This combination addresses both short-term discomfort and long-term osteoarthritis (OA) management.
Compared to other HA-only treatments, such as Synvisc or Durolane, Cingal injections offer a more comprehensive approach. While traditional viscosupplements focus solely on enhancing joint function and mobility, Cingal delivers quicker relief from inflammation and pain, making it especially beneficial for patients experiencing acute OA flare-ups.
Indications and Recommended Usage
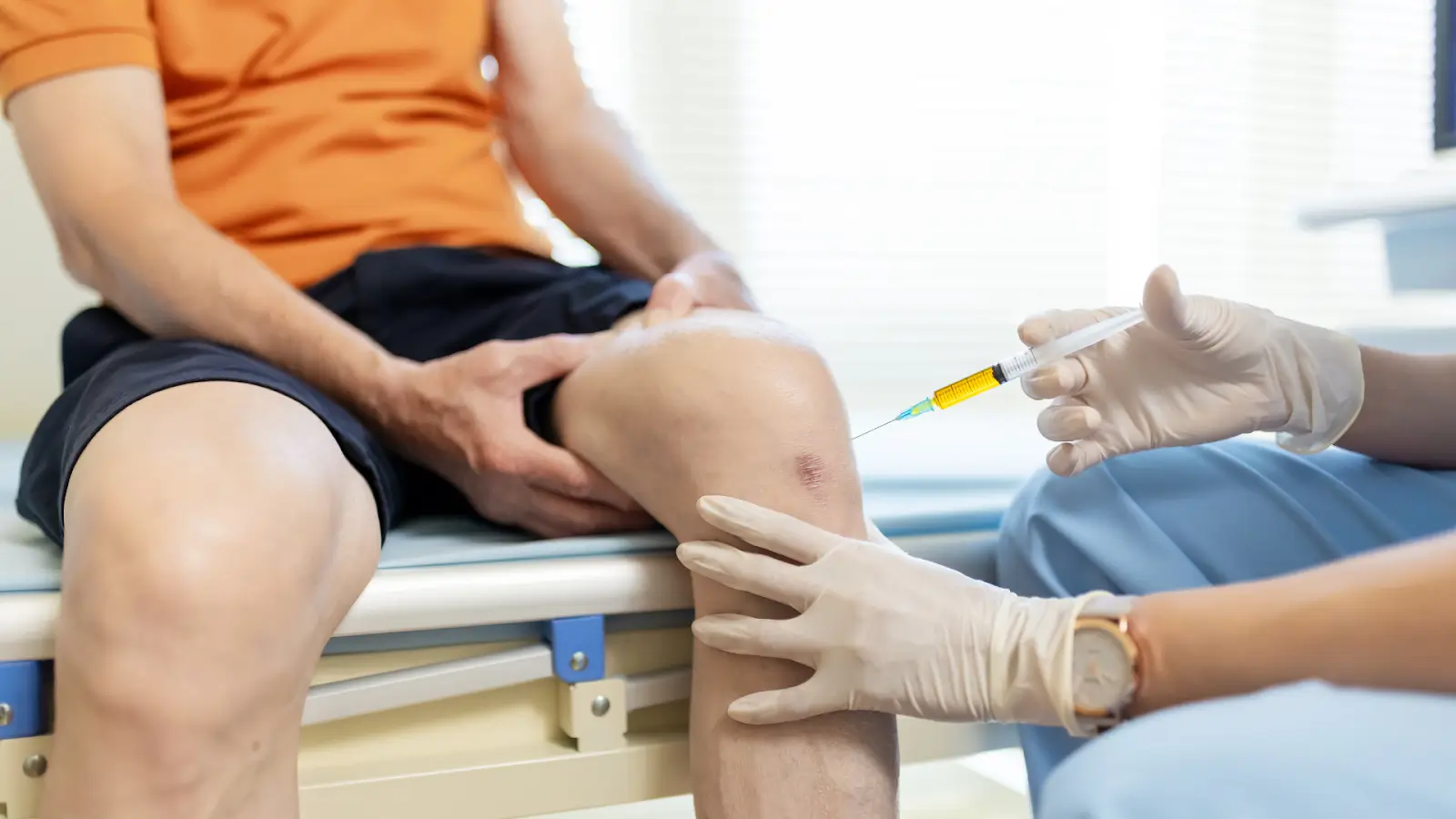
Cingal targets knee osteoarthritis (OA) in patients who experience persistent pain and limited mobility despite other non-surgical treatments, such as oral pain relievers or physical therapy. Thanks to its dual-action formulation combining hyaluronic acid and a corticosteroid, it is particularly beneficial for individuals dealing with moderate to severe OA or acute flare-ups.
Recommended Usage
- Administration: Cingal is administered as a single intra-articular injection directly into the knee joint.
- Dosage: One syringe containing 4 mL of the solution is typically sufficient for immediate and long-lasting relief.
- Treatment Duration: Relief from symptoms can last up to six months, although results may vary based on the individual’s condition and activity level.
Patients should consult their healthcare provider to determine if Cingal is the right treatment for their OA symptoms. Healthcare providers should consider factors such as joint health, previous treatments, and medical history to ensure safety and optimal outcomes.
Conclusion
Cingal combines the benefits of a viscosupplement and a corticosteroid, providing a dual-action solution for knee osteoarthritis pain. While it has successfully completed phase III trials and received approval in Canada and Europe, its FDA approval in the United States remains pending.
This leaves its availability in the U.S. uncertain for now, but its innovative formulation and demonstrated efficacy suggest significant potential for future use in managing osteoarthritis symptoms.
FAQs
1. What is Cingal for?
Cingal treats knee osteoarthritis by reducing pain and improving joint mobility.
2. How does Cingal work?
Cingal combines hyaluronic acid for joint lubrication and a corticosteroid for immediate pain and inflammation relief, offering both short- and long-term benefits.
3. Has the FDA approved Cingal in the United States?
No, Cingal has not yet received FDA approval for use in the U.S., though it is approved in over 35 other countries.
References
Pereira TV, Jüni P, Saadat P, et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. BMJ. Published online July 6, 2022:e069722. doi:10.1136/bmj-2022-069722
Bert J, Kenney J, Sgaglione NA, et al. Viscosupplementation for osteoarthritis of the knee: A key Opinion Leader panel discussion. Journal of Managed Care & Specialty Pharmacy. 2018;24(6-a Suppl):S2-S8. doi:10.18553/jmcp.2018.24.6-a.s2
Anika Therapeutics. (n.d.). Cingal. Retrieved November 18, 2024, from https://anika.com/medical/products/cingal/
Products
Cart
Log In
Newsletter
Subscribe for exclusive offers and updates on new arrivals
Share feedback at:
Working Hours
Monday to Friday: 9 AM to 6 PM EST
The Most Popular Brands
Med Supply Solutions
Support
Copyright 2025. Med Supply Solutions