Business Hours: Monday - Friday: 9 AM - 6 PM EST
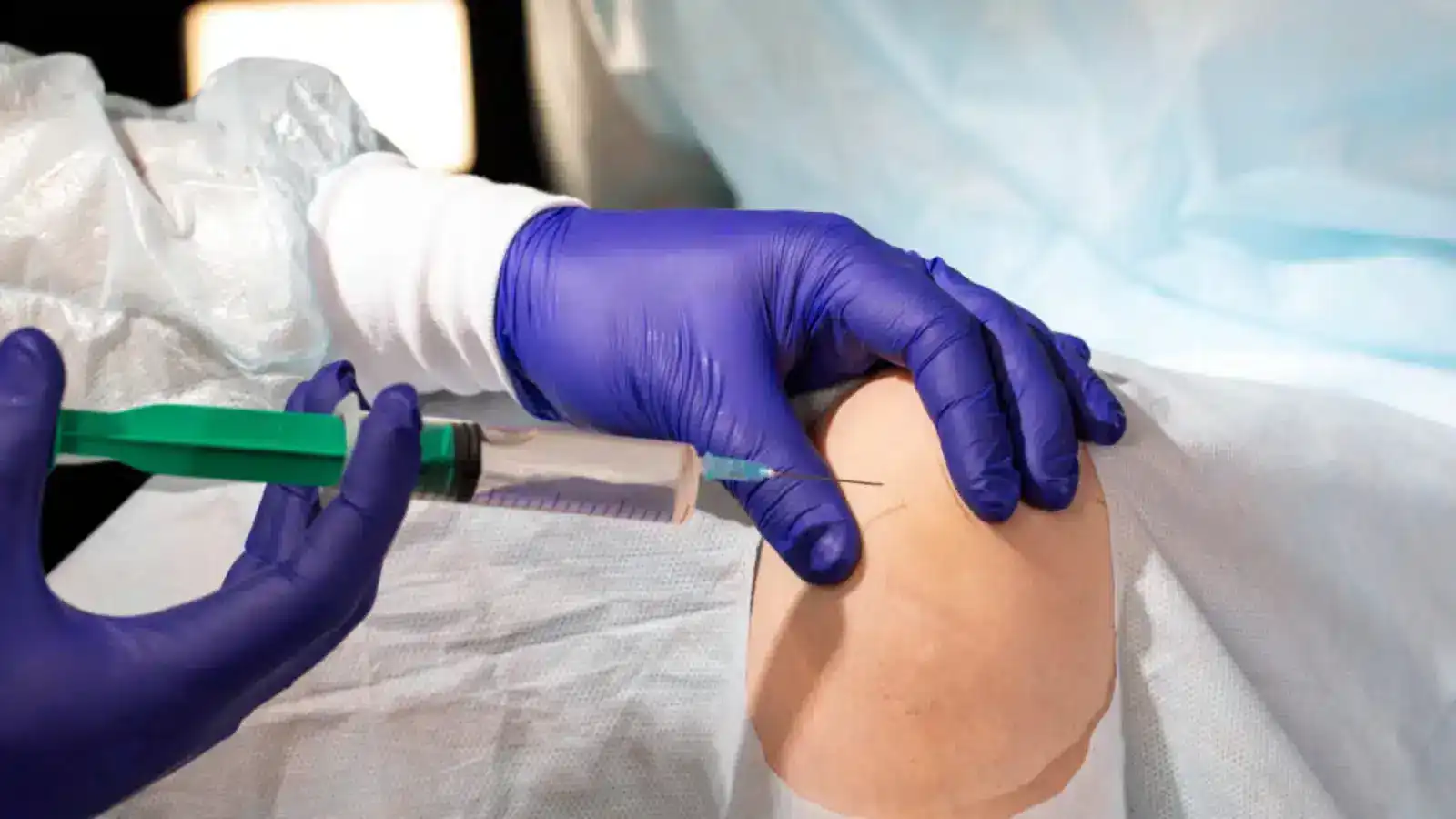
Crespine Gel Benefits – Why It Works for Joint Treatments
David Fuller
Last Updated On: December 30, 2024
Knee osteoarthritis (OA) is one of the most common causes of joint pain, affecting over 32.5 million adults in the United States. Treatments typically aim to relieve pain and improve joint function, with injectable therapies gaining popularity for their targeted and minimally invasive approach.
Crespine Gel is a cutting-edge injectable solution designed to address knee OA symptoms and support skin health. Combining hyaluronic acid with other active ingredients provides enhanced joint lubrication and pain relief, making it a versatile choice for managing joint pain caused by conditions like osteoarthritis.
In this article, we will explore the benefits and potential drawbacks of Crespine Gel for joint treatments, helping you determine if it’s the right option for your needs.
Key Takeaways
- Crespine Gel provides pain relief, reduces joint inflammation, and enhances mobility, effectively managing osteoarthritis.
- The gel’s primary component, hyaluronic acid, restores lubrication and cushioning in joints, ensuring smooth movement and long-lasting effects.
- As a non-surgical option, Crespine Gel offers patients a less invasive alternative with sustained benefits and reduced treatment frequency.
- Medical professionals can utilize Crespine Gel to deliver targeted care, focusing on patient outcomes and adherence to regulatory guidelines.
About: Med Supply Solutions has been operating since 2016 and is known as one of the industry’s top and trusted suppliers of cosmetic and viscosupplementation products. Contact our sales department for more information about buying Crespine online.

CRESPINE® GEL PLUS
CROSS-LINKED HA
NON-CROSSLINKED HA
PRILOCAINE
$139.00
Tier pricing
Save 3.6%
3 or more
$134.00 each
Save 7.19%
11 or more
$129.00 each
Save 8.63%
21 or more
$127.00 each
What is Crespine Gel?

Crespine Gel is a viscosupplement that can alleviate pain and inflammation associated with osteoarthritis. Its key component is hyaluronic acid, also known as sodium hyaluronate, which is naturally found in the body’s connective tissues, skin, joints, and eyes. Hyaluronic acid is renowned for its remarkable water-retaining properties, helping to keep tissues well-hydrated.
In skin rejuvenation treatments, hyaluronic acid is used for its ability to deeply hydrate the skin, reduce the appearance of wrinkles, and improve skin elasticity. When injected into the skin, it acts as a filler, providing immediate plumping effects and promoting collagen production for long-term benefits.
Benefits of Crespine Gel for Joint Treatments

Crespine Gel offers significant advantages for individuals managing joint conditions like osteoarthritis. Its primary benefit lies in the ability to alleviate pain and improve joint function by replenishing hyaluronic acid, which often depletes in affected joints.
- Treatment of Osteoarthritis: Crespine Gel works by acting as a viscosupplement, restoring lubrication and cushioning within the joint. This reduces friction between bones, alleviating pain and inflammation commonly associated with osteoarthritis.
- Improved Joint Mobility: By enhancing joint lubrication, Crespine Gel enables smoother movement and reduces stiffness, allowing patients to regain mobility and perform daily activities with greater ease.
- Long-Term Benefits: Unlike temporary solutions, Crespine Gel’s unique formulation provides prolonged effects. Patients often experience relief lasting several months, reducing the frequency of treatments while improving overall joint health.
Crespine Gel stands out as an effective, minimally invasive option for managing joint conditions. It offers both immediate and sustained relief for a better quality of life.
How Medical Professionals Can Utilize Crespine Gel in Practice
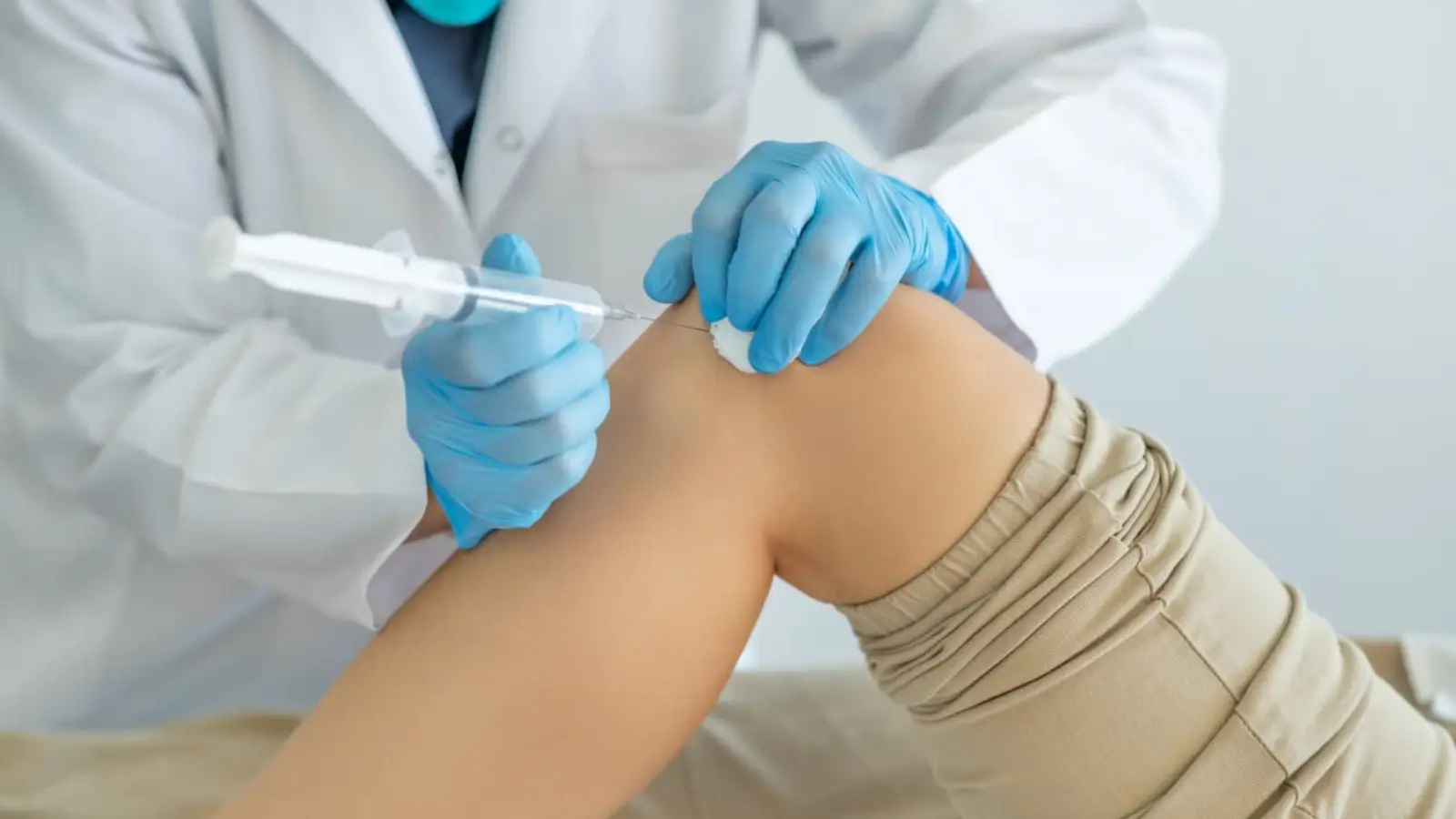
Medical professionals can effectively utilize Crespine Gel in their practice to provide advanced care for patients with knee osteoarthritis and other joint conditions.
- Joint Injections for Pain Relief: Crespine Gel is designed for intra-articular injections, offering targeted pain relief by replenishing hyaluronic acid levels within the joint. This helps reduce friction, alleviate inflammation, and improve joint mobility, enabling patients to regain their quality of life.
- Enhancing Patient Outcomes: With its long-lasting effects, Crespine Gel minimizes the frequency of treatments while providing sustained relief. This reduces the burden on patients and enhances overall satisfaction, making it a reliable choice for managing joint pain.
Practitioners should remain informed about Crespine Gel FDA approval status, as it is not yet authorized for use in the United States. Adhering to regulatory guidelines is essential for ensuring patient safety and compliance with local laws.
Conclusion
Crespine Gel is a cutting-edge injectable therapy offering significant benefits for individuals with knee osteoarthritis. By replenishing hyaluronic acid in joints, it reduces pain, enhances mobility, and improves quality of life. Its long-lasting effects and non-invasive nature make it an attractive option for both patients and medical professionals.
However, understanding the regulatory status, such as the lack of FDA approval in the United States, is essential when considering this treatment. By staying informed and adhering to established guidelines, healthcare providers can safely and effectively incorporate Crespine Gel into their practice to meet patient needs.
FAQs
1. How does Crespine Gel work?
It contains hyaluronic acid, which replenishes natural joint lubrication, reduces inflammation, and enhances joint movement by minimizing bone friction.
2. How long do the effects of Crespine Gel last?
The Crespine Gel benefits typically last up to six months, depending on the severity of the condition and the individual’s response to treatment.
3. Is Crespine Gel safe to use?
Crespine Gel is safe especially when a healthcare professional administers the treatment. Typical Crespine Gel side effects are generally mild, including temporary swelling or redness at the injection site.
4. Does Crespine Gel have FDA approval?
No, Crespine Gel has not yet received FDA approval for use in the United States. However, it has certification in several other regions, including parts of Europe and Asia.
References
OA Prevalence and burden. Osteoarthritis Action Alliance. https://oaaction.unc.edu/oa-module/oa-prevalence-and-burden/
Bashaireh, K., Naser, Z., Hawadya, K. A., Sorour, S., & Al-Khateeb, R. N. (2015)The efficacy of cross-linked osteoarthritis single injection on knee osteoarthritis: a post-marketing Phase IV study. Drug design, development, and therapy, 9, 2063–2072. https://doi.org/10.2147/DDDT.S81524
Products
Cart
Log In
Newsletter
Subscribe for exclusive offers and updates on new arrivals
Share feedback at:
Working Hours
Monday to Friday: 9 AM to 6 PM EST
The Most Popular Brands
Med Supply Solutions
Support
Copyright 2025. Med Supply Solutions